A Step Beyond The Basics
In the last Flight School segment we discussed the basics of batteries – key terms and what they mean. Now we are going to dive in a bit deeper and discuss the different chemistries that are out there and some of the differences between them.
All batteries, as explained previously, consist of a cathode, an anode, and an electrolyte. Through a chemical reaction (a redox reaction specifically), electrons flow through these parts creating an electrical current, which is then used to power any device that is connected to the battery circuit. The chemical makeup of the cathode, anode, and electrolyte can change aspects of the battery. This makes understanding battery chemistry a must when searching for the right battery for your airplane.
Lets take a look at the properties that will differ based on battery chemistry:
Cell Voltage - Battery voltage is a measure of the pressure from a power source that moves charged electrons (known as current) through a circuit. It is measured in volts (V). Batteries of the same chemistry will provide the same nominal voltage, but that nominal voltage will differ when comparing batteries of different chemistries.
Energy Density - Energy density is the ratio of energy stored to size of the battery cell. This can come into play when considering fuselage size and flight style. Energy density is energy stored per unit of volume, gravimetric energy density is energy per unit of mass.
Rechargeability - Some batteries are not rechargeable, and others are. The battery chemistries you will see in the RC flight industry will be rechargeable.
Self-Discharge - Even if you are not using a battery, it will still undergo chemical reactions that will result in a loss of charge. This process is known as self-discharge. Depending on the chosen battery chemistry, the amount of self-discharge over time can vary.
Cycle Life - In addition to self-discharge, batteries have a limit on the amount of times it can be charged and discharged. This is called cycle life, and again, differs based on chemistry.
Economics - Just like everything in life, based on the material inside, the cost of your batteries can be effected.
Safety - Some chemicals are very stable, others are very volatile. Battery chemistry plays a big part in how safe your battery is, and what practices should be employed.
Now that we have discussed some of the properties that can be effected by different battery chemistries, lets take a look at the chemistries found in RC Flight.
Nickel Based Batteries; (NiCd and NiMH)
NiCd – Nickel-Cadmium batteries are made with a Nickel Hydroxide (Ni(OH)2) anode, Cadmium Hydroxide (Cd(OH)2) Cathode, and an electrolyte of Potassium Hydroxide (KOH). While they are affordable, provide a large cycle life, and can charge quickly, they have a high self discharge rate and it is very hard to tell charge based on voltage. In addition, they are toxic and corrosive, so disposal is difficult. NiCd batteries also suffer from the “memory effect” which is where a battery, if not fully discharged, will “remember” the previous discharge level, loosing the ability to discharge past that level in the future.
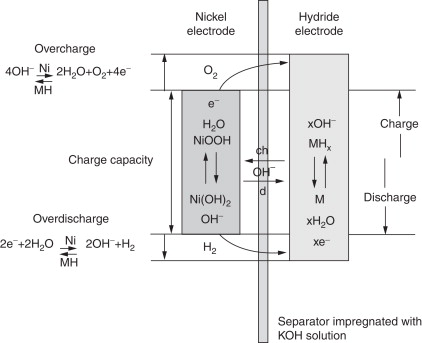
NiMH – Nickel Metal Hydride offer many of the same pros and cons as NiCd, only it removes the toxic Cadmium and replaces it with a metal alloy hydride for the anode (which actually results in higher performance).
Lithium Based Batteries; (Li-Ion, LiPo, LiFe)
Lithium based batteries have high energy densities, making them a great choice when weight and size are of importance. In addition, they also have much lower self-discharge rates.
Li-Ion – These batteries, Lithium Ion, use a porous material for the anode (such as graphite or silicone) and an ionic lithium compound for the cathode. Multiple chemistries exist for the cathode, including Lithium Cobalt Oxide (LiCoO2), Lithium Manganese Oxide (LiMn2O4), and Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2). The electrolyte used consist of lithium salts dissolved in an organic compound, which due to the properties of lithium as well as the compound, are highly explosive. These batteries come in a hard outer casing.
LiPo – Lithium Polymer batteries are much like the Lithium-Ion, however they differ in what the electrolyte is made of. In LiPo batteries, you will find a gel lithium polymer instead of a liquid. This provides less of a chance of the electrolyte leaking out. Although this is a safety improvement, LiPo batteries have a soft casing as opposed to their counterparts – Li-Ion.

LiFePO4 – Lithium Iron Phosphate batteries are a type of Lithium Ion battery, but this particular chemistry has its own set of properties, differing from the other Lithium Chemistries. The energy density of LiFe batteries comes in a bit lower than that of the Li-ion or LiPo batteries, but better than that of the Nickel based batteries. In addition, LiFe batteries have a long cycle-life, are more affordable and safer overall.
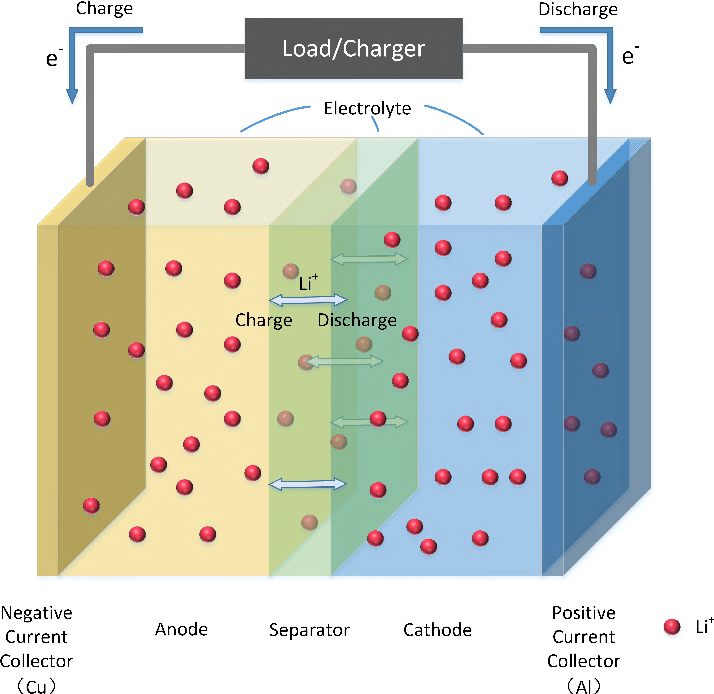
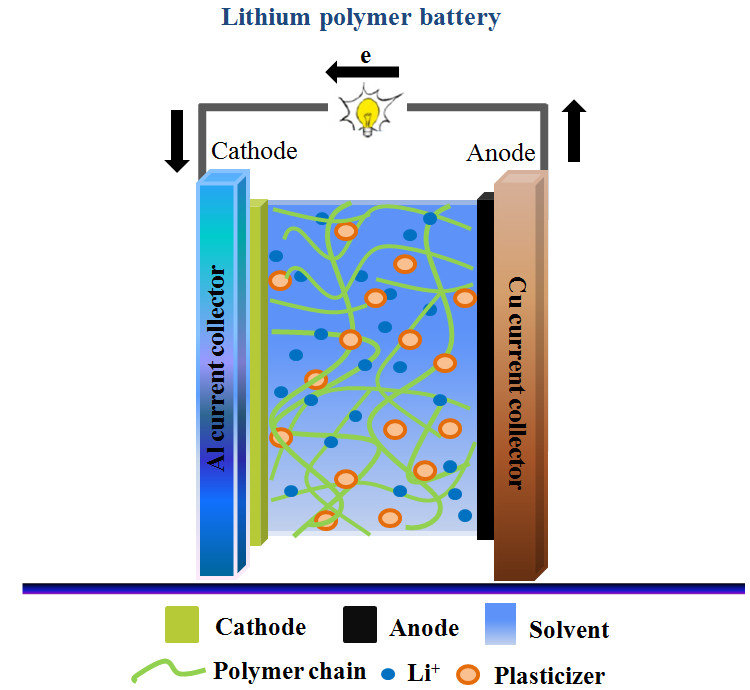
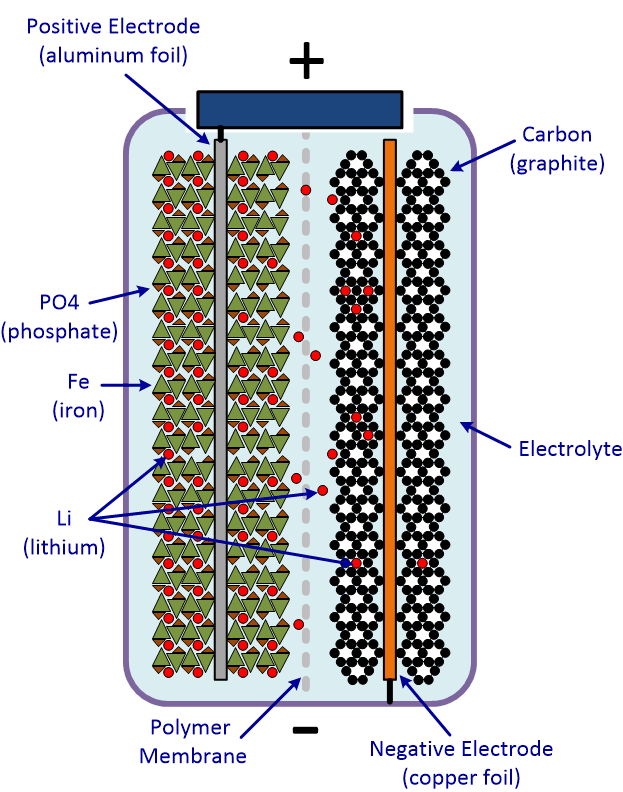
Comparison Chart: